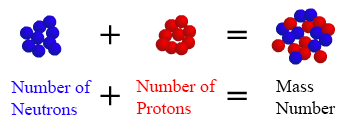
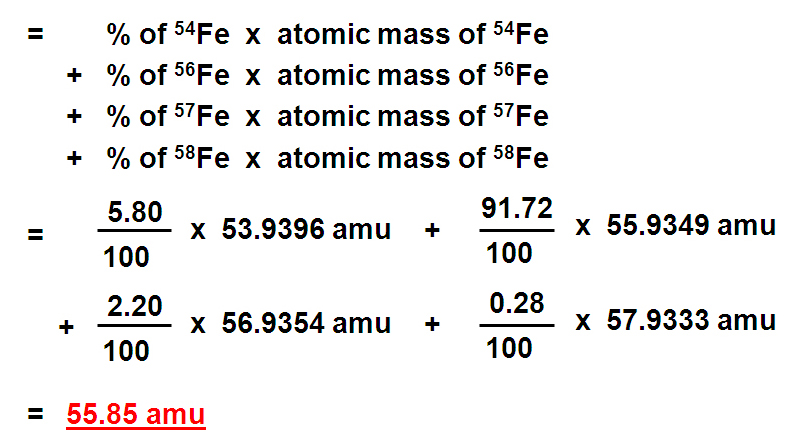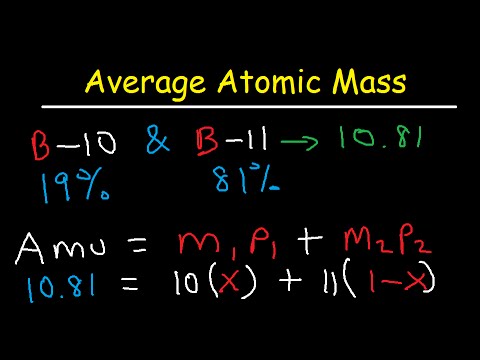Outstanding Tips About How To Get Atomic Mass
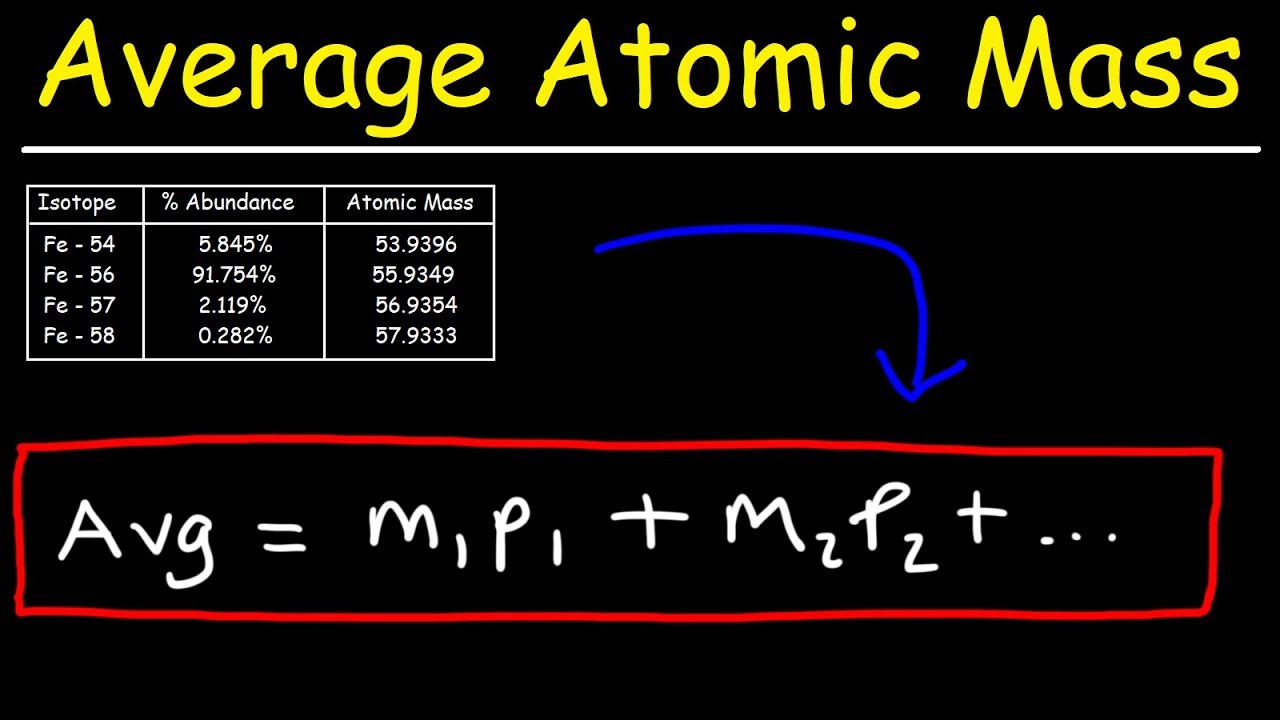
For each isotope, multiply its mass by the percent.
How to get atomic mass. For example, the atomic mass of lithium is 6.941 da. Identify the percentage of each isotope in the composition of the element and its mass. Thus, atomic mass of oxygen = 15.995 amu (99.76/100) + 16.999 amu (.04/100) + 17.999 amu (.2/100) = 15.956612.
The atomic mass is used to calculate the average mass of molecules and elements. This chemistry video tutorial explains how to calculate the average atomic mass of an element given the percent abundance of each isotope.my website: It is also used to solve.
Sum the result to get the atomic mass of the element; On the basis of the abundance of isotopes, we can calculate the isotopic mass and average atomic mass of an element. The average atomic mass can be calculated by multiplying the mass number and natural abundance of each of the isotopes and then adding them all together.
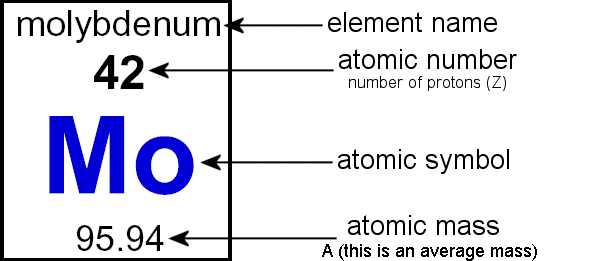
The atomic mass of an element is commonly given in grams per mole. To calculate it, we add up all these particles’ masses: How to calculate the atomic mass of an atom a specific isotope’s atomic mass corresponds to its total mass expressed in dalton (u), also called unified atomic mass units.
One proton equals 1,836 electrons. The atomic mass is composed of the protons, neutrons and electrons in an atom. Boron isotope a has 10.









/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)